Introduction
Chemical bonding and molecular structure form the backbone of modern chemistry. Ever wondered why water is liquid while carbon dioxide is gas at room temperature? The answer lies in understanding chemical bonds and molecular structures.
Kossel and Lewis Approach
The journey of chemical bonding theories began with the pioneering work of Kossel and Lewis. Kossel proposed the ionic bonding model, while Lewis introduced the concept of electron sharing.
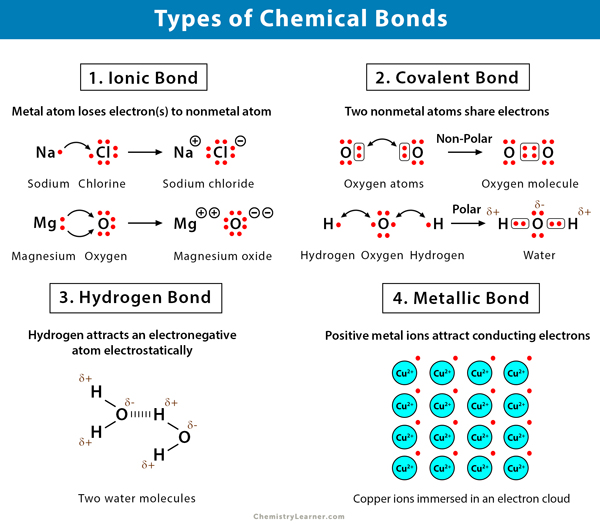
Types of Chemical Bond
Chemical bonds are categorized based on how atoms interact:
- Ionic Bonds: Ionic bonds involve the transfer of electrons from one atom to another, creating oppositely charged ions. For example, NaCl forms through sodium donating an electron to chlorine.
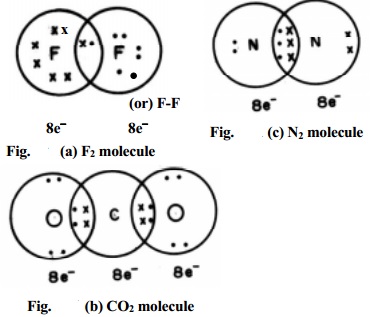
- Covalent Bonds: In covalent bonding, atoms share electron pairs. Molecules like H₂ and O₂ showcase this sharing mechanism.
- Metallic Bonds:Metallic bonds occur in metals, where electrons flow freely among a lattice of positive ions, imparting conductivity and malleability.
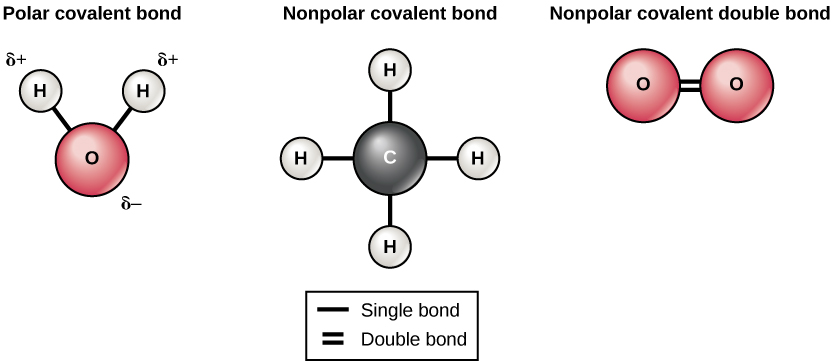
Covalent Bond
Covalent bonds are formed when atoms share electrons to achieve stability. This type of bond is prevalent in organic molecules and most non-metals.
For instance, in a water molecule (H₂O), oxygen shares electrons with hydrogen atoms, creating strong covalent bonds. Unlike ionic bonds, covalent bonds lead to the formation of discrete molecules.
What makes covalent bonds fascinating? Their versatility! They allow for the formation of simple molecules like methane (CH₄) to complex structures like DNA.

Coordinate Bond
Coordinate bonds are a special type of covalent bond where both electrons in the shared pair come from the same atom. This bonding occurs in compounds like ammonium ion (NH₄⁺), where nitrogen donates a lone pair to bond with a hydrogen ion.

Bond Parameters
Bond parameters like bond length, bond angle, and bond enthalpy help predict molecular behavior.
Understanding bond parameters helps predict the behavior of molecules:
1. Bond Length
The distance between the nuclei of two bonded atoms. Shorter bond lengths typically indicate stronger bonds.
2. Bond Angle
This parameter defines the spatial arrangement of atoms in a molecule, influencing its geometry and reactivity.
3. Bond Enthalpy
A measure of bond strength, bond enthalpy determines the energy required to break a bond.
These parameters are essential for understanding molecular stability and reactivity.

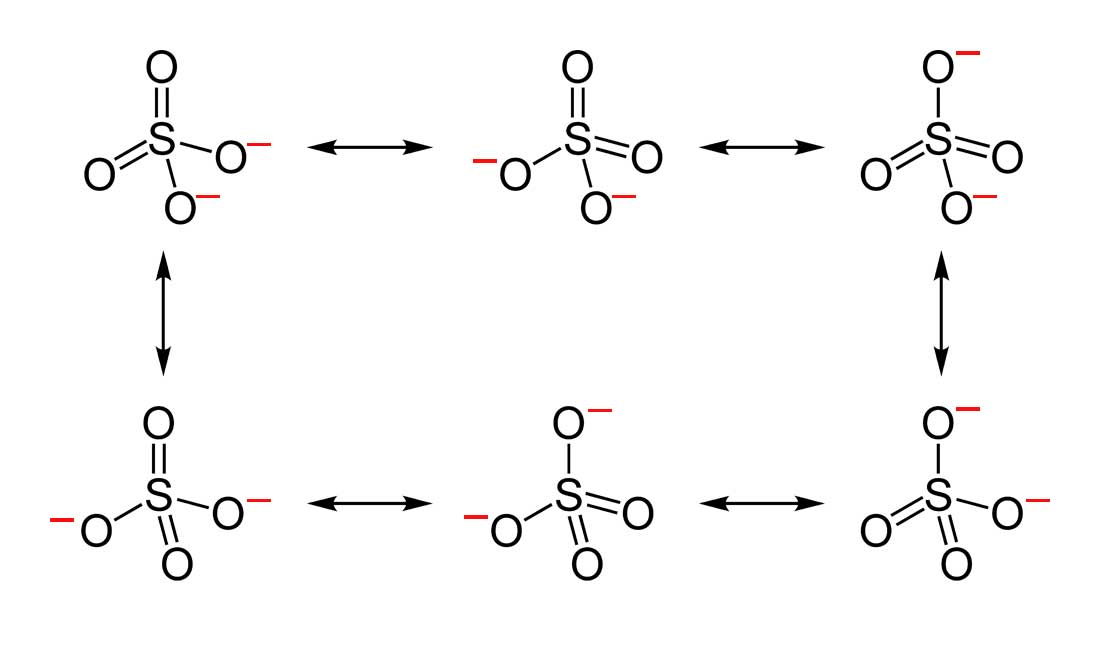
Resonance
Resonance occurs when electrons delocalize, stabilizing molecules like benzene.
Resonance is a fascinating concept that explains why some molecules can’t be adequately represented by a single Lewis structure. Instead, these molecules exist as a hybrid of multiple possible structures, known as resonance structures.
For example, in the carbonate ion (CO₃²⁻), the double bond between carbon and oxygen isn’t fixed in one location. Instead, it "resonates" between the three oxygen atoms, resulting in equal bond lengths and enhanced stability.
This delocalization of electrons not only stabilizes molecules but also affects their chemical properties. Resonance is key to understanding phenomena like aromaticity in benzene and the behavior of conjugated systems.
Polarity and Dipole Moment
Molecular polarity determines how a molecule interacts with electric fields, other molecules, and solvents. Polarity arises from the unequal sharing of electrons between atoms due to differences in electronegativity.
For instance, water (H₂O) is polar because oxygen pulls electrons more strongly than hydrogen, creating a dipole moment. This is why water dissolves ionic and polar substances efficiently.
The dipole moment, measured in Debye units, quantifies the polarity of a molecule. Molecules with higher dipole moments have stronger intermolecular forces, influencing boiling points, solubility, and reactivity.

Polarization
Polarization occurs when an external electric field distorts the electron cloud of an atom or molecule. This distortion creates temporary dipoles, which affect the chemical and physical properties of substances.
Fajan’s Rules help predict the extent of polarization in ionic compounds. For example, smaller, highly charged cations like Al³⁺ polarize anions more effectively, leading to partial covalent character in bonds.
Polarization explains why some compounds exhibit unexpected properties, such as higher melting points or unusual reactivity patterns. Understanding it is crucial for predicting chemical behavior in real-world applications.
VSEPR Theory
The Valence Shell Electron Pair Repulsion (VSEPR) Theory is a simple yet powerful model for predicting molecular shapes. It states that electron pairs around a central atom arrange themselves to minimize repulsion.
For instance:
- A molecule with two bond pairs, like CO₂, adopts a linear geometry.
- In water (H₂O), two lone pairs push the bond pairs, creating a bent shape.
By applying VSEPR theory, chemists can predict the 3D structure of molecules, which is critical for understanding their interactions and functions in chemical and biological systems.
Hybridisation
Hybridisation explains how atomic orbitals mix to form new hybrid orbitals, leading to the observed molecular geometries.
Types of Hybridisation:
- sp Hybridisation: Found in linear molecules like BeCl₂.
- sp² Hybridisation: Present in trigonal planar structures, such as BF₃.
- sp³ Hybridisation: Seen in tetrahedral molecules like CH₄.
The type of hybridisation determines bond angles, bond strength, and molecular shape. For example, the 109.5° bond angle in methane results from sp³ hybridisation.
By mastering this concept, students can better visualize and explain the molecular architecture of compounds.

Valence Bond Approach of Covalent Bond
The Valence Bond Theory (VBT) describes covalent bonding through the overlap of atomic orbitals. When orbitals overlap, they form a bond, releasing energy and stabilizing the system.
For example:
- The overlap of two s orbitals forms a sigma (σ) bond.
- A pi (π) bond arises from the sideways overlap of p orbitals.
VBT emphasizes the quantum mechanical nature of bonding and provides insight into the strength and orientation of bonds. It complements hybridisation theory by offering a detailed view of bond formation.
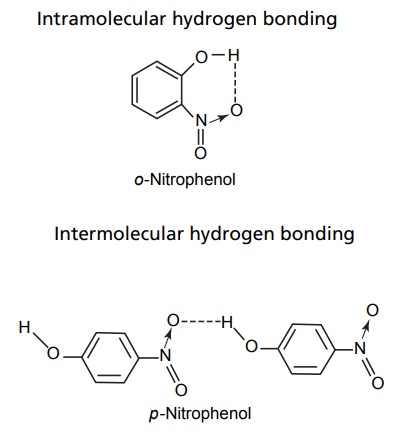
Hydrogen Bonding
Hydrogen bonding is a special type of intermolecular force that occurs when hydrogen, bonded to a highly electronegative atom (like N, O, or F), interacts with another electronegative atom.
There are two types:
- Intermolecular Hydrogen Bonding: Found in water, leading to its high boiling point.
- Intramolecular Hydrogen Bonding: Occurs within a molecule, as seen in ortho-nitrophenol.
This bond plays a crucial role in biological systems, stabilizing DNA structures and determining protein folding. Without hydrogen bonding, life as we know it wouldn’t exist.
Conclusion
Chemical bonding and molecular structure provide a foundation for understanding the diversity of matter in our universe. By grasping the concepts of ionic and covalent bonds, resonance, polarity, and hybridisation, students can decode the mysteries of chemistry.
These principles are not just theoretical—they are the key to innovations in medicine, materials science, and energy. Embracing these ideas opens a gateway to discovering the fundamental workings of nature.
FAQs
1. What is the significance of chemical bonding in daily life?
Chemical bonding explains the structure and properties of substances, from water’s liquid nature to the strength of steel. It is foundational to understanding chemical reactions and material behavior.
2. How does VSEPR theory predict molecular shape?
VSEPR theory uses electron pair repulsion to determine the spatial arrangement of atoms. For example, linear, trigonal planar, and tetrahedral geometries result from different electron pair configurations.
3. What are resonance structures, and why are they important?
Resonance structures represent the delocalization of electrons in molecules like benzene. They stabilize molecules and influence their chemical properties.
4. Why is hybridisation crucial in chemistry?
Hybridisation explains molecular geometry and bonding patterns, making it easier to predict the behavior of compounds like methane, ethene, and ammonia.
5. What role does hydrogen bonding play in nature?
Hydrogen bonding stabilizes biological macromolecules like DNA and proteins, influences water's properties, and is essential for life processes.
Comments
Post a Comment